Project List
PRiSMA
Pregnancy Risk Stratification Innovation and Measurement Alliance for Maternal and Newborn Health.

Implementation Duration:
(2022 - 2025)
PROJECT OVERVIEW
ARC aims to generate robust evidence and address the gaps in implementation research around maternal and newborn health, with emphasis on pregnancy risk factors and their associations with adverse pregnancy outcomes, including stillbirth, neonatal mortality and morbidity, and maternal mortality and severe morbidity. The goals are to develop a harmonized data set to improve the understanding of pregnancy risk factors, vulnerabilities, and morbidity and mortality and to estimate the burden of these risk factors and outcomes in LMICs. Ultimately, these data will inform the development of innovative strategies to optimize pregnancy outcomes for mothers and their newborns. We aim to collect data on 6000 pregnant women over a three-year period.
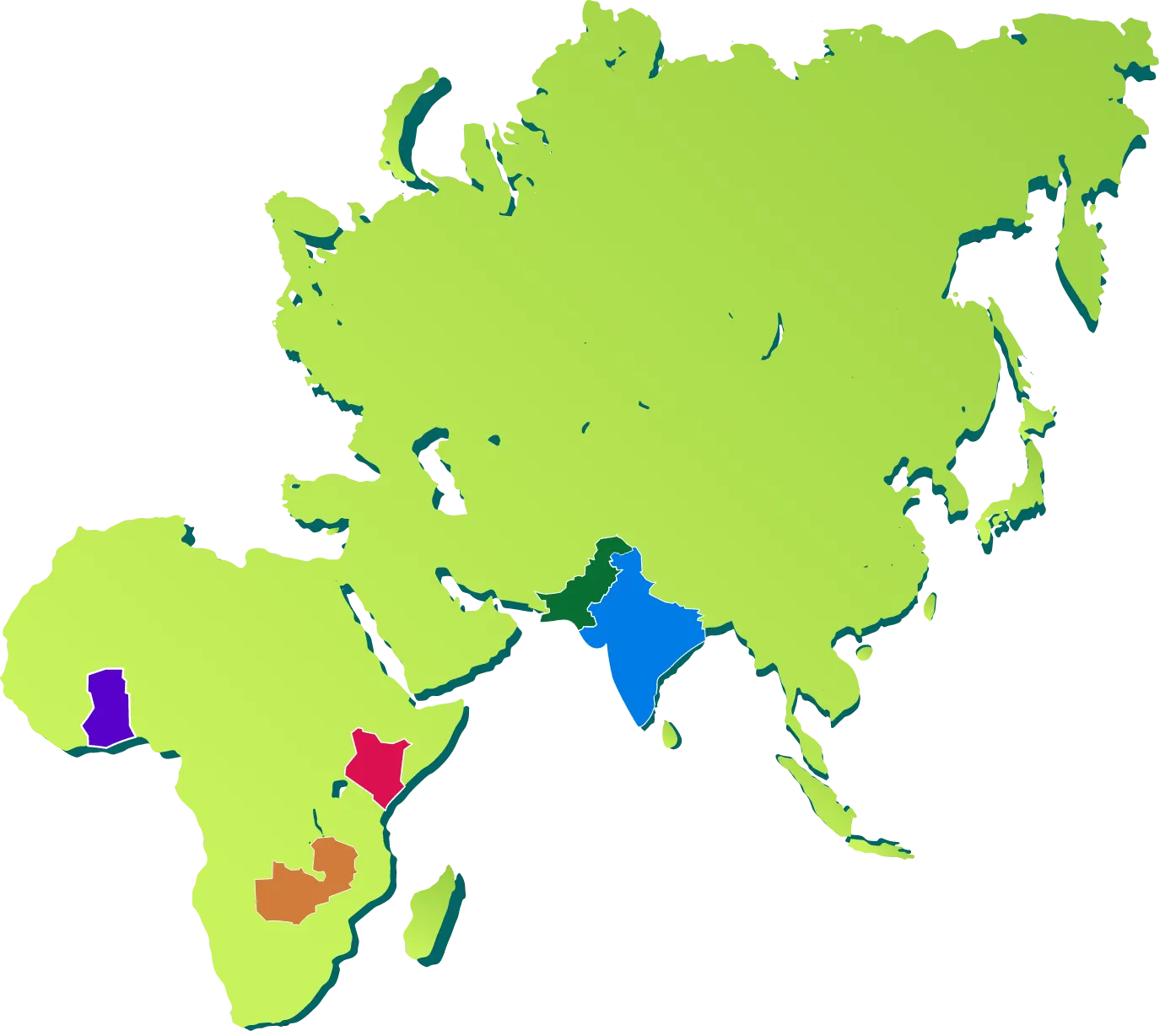
PAKISTAN
Rehri Goth & Ibrahim Hyderi
INDIA
Haryana & Asam
KENYA
Kisumu & Siaya Counties
ZAMBIA
Lusaka
GHANA
Kintampo
The PRiSMA MNH study is a prospective, open cohort study with five countries including sites from India (Haryana and Assam), Kenya (Kisumu and Siaya counties), Ghana (Kintampo), Zambia (Lusaka), and Pakistan (Rehri Goth and Ibrahim Hyderi).
PROJECT CONCENTRATED AREAS
The Study is conducted in Ibrahim Hyderi and Rehri Goth


PROJECT OBJECTIVES

Enteric Pathogen Control in Karachi
A civil society coalition to strengthen immunization access.

Implementation Duration:
2021 - Till Date
PROJECT OVERVIEW
Karachi is a large metropolitan area of Pakistan with a population of ~20 million. Nearly half of Karachi’s population lives in 988 slum areas, of which only 6% of these slums are officially registered or legally regularized. Administratively, the mega-city is divided into six districts, which are broken down into 18 towns, and further divided into 178 union councils and 10 cantonment areas. People of different linguistic and religious origins from different parts of the country migrate to Karachi for economic, educational, and political opportunities. Karachi has poor routine immunization coverage at 55% of children under the age of 5 fully immunized (FIC). MICS survey of Karachi (2014) and PDHS survey findings of the Sindh Province (2017-18) show a slight increase in FIC with basic vaccines in the urban areas. Immunization coverage is particularly low in 8 union councils, which include Gujro, Songal, Manghopir, Ittehad town, Chisti Nagar, Islamia Colony, Muzaffarabad, and Muslimabad. In this proposal, we will refer to these areas as Disease Hotspot Areas.

PROJECT CONCENTRATED AREAS
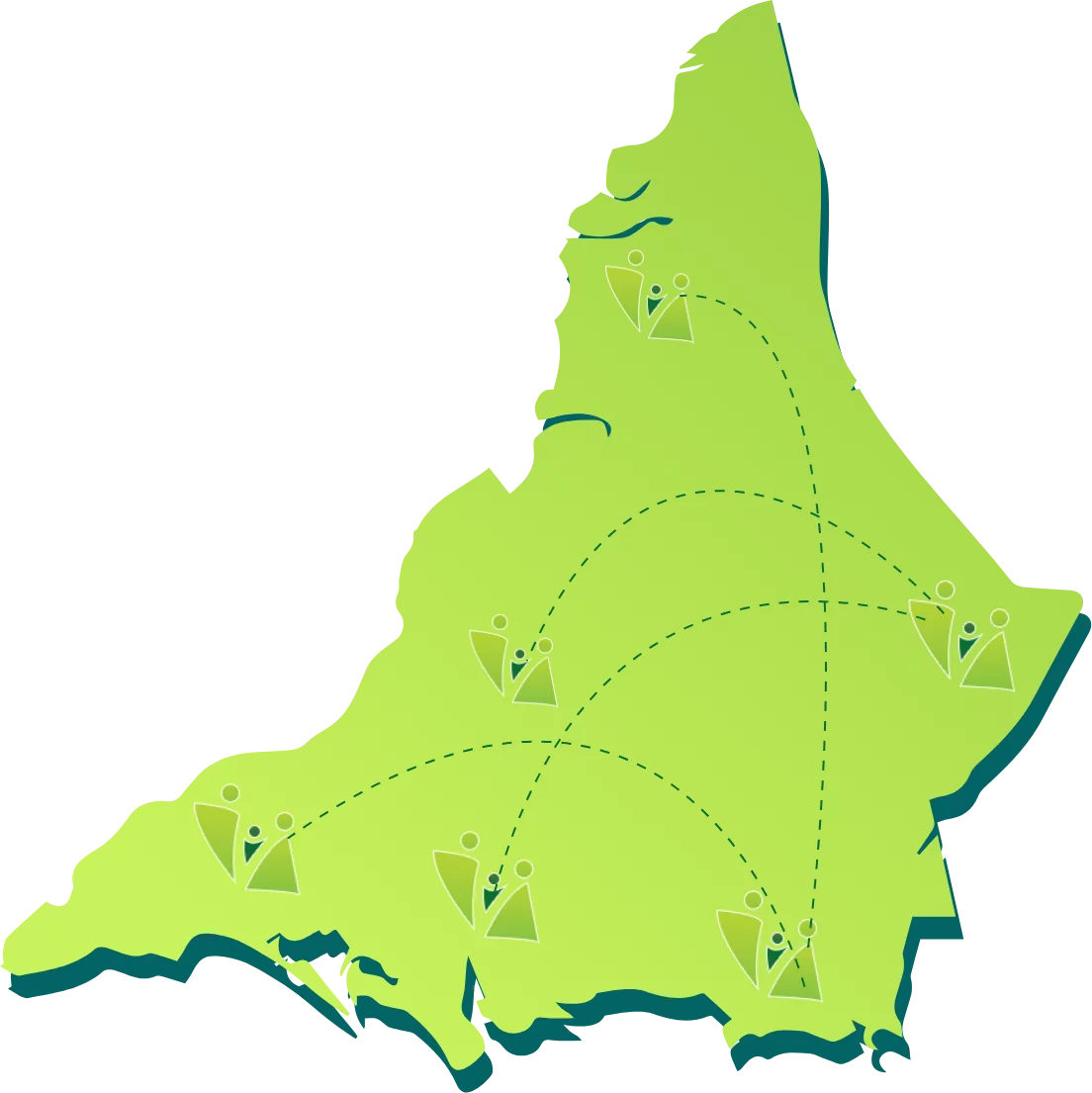
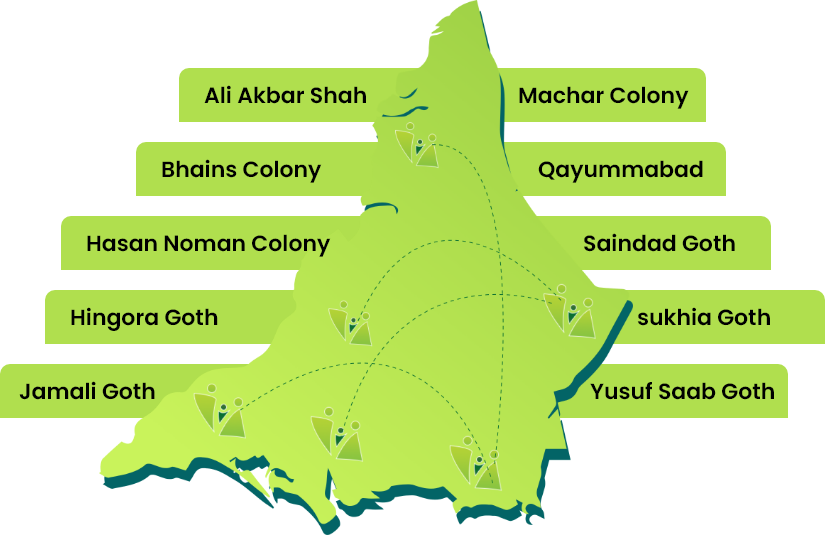
Malir
AIi Muhammad Jokhiyo, Saleh Muhammad Goth, Jumma Goth, Yousuf Arfani Goth, Majeed Colony Metroville 2, Sherpao Colony Lalaabad, Ghaggar Patak, Rehri Goth, Bhains Colony, Ibrahim Hyderi, ED – Bilal Colony, ED – Gulshan e Bunair.
West
Khairabad Behar Colony, Yousuf Goth 4K, Khair Muhammad Goth.
East
Yousuf Sahab Goth, Bhitataiabad Airport, Saidad Goth, Hassan Noman Colony, Machar Colony Gujro, Qayummabad – KGH, Jamali Goth, Sukhiya Goth, Hingora Goth.
KORANGI
Bilal Colony, Mehran Town, Ittehad Colony, Ali Akbar Shah.
Central
Paposh Graveyard, Zareena Colony.
Kemari
Muhammad Khan Colony, Kokan Colony, Moach Goth, Mewashah Golimaar, Shireen Jinnah Sultan Abad, Machar Colony, SGD – Muhammad Khan Ittehad Town, SGD – Ittehad Town Block D.
PROJECT OBJECTIVES

Integrated Research Platform
(Digital Health at Scale)
VITAL Pakistan has launched this research platform to generate high-quality, multi-dimensional, and longitudinal data at scale for maternal & child health (MNCH) in low- middle-income countries (LMICs).

Implementation Duration:
Three (3) Years
PROJECT OVERVIEW
It is critical to establish trends of the burden of disease, facilitate the development of contextually appropriate guidelines, and test relevant interventions. However, establishing disease burden estimates is often resource intensive and marred by limitations such as reliance on self-reported data. In addition, there is also a need to reduce the lag between the generation of knowledge or evidence and translation into practice. Robust electronic healthcare records have the potential to overcome these limitations. Through prior investments by BMGF, VITAL Pakistan Trust has developed and implemented a unique, integrated digital platform for front-line healthcare workers.
Study design
Prospective cohort study.
Women seeking antenatal and/or postnatal care services who consent to be enrolled in the study.
All women who seek antenatal and/or postnatal care services at any partner clinic in our catchment population will be eligible for the study. Data on key variables (e.g., socio-demographics, pregnancy history, risk factors, maternal morbidity, birth outcomes, infant danger signs, anthropometry, and EBF practices) will be collected through the integrated digital platform during the antenatal and postnatal care periods.
Benefits to society
The project will expand antenatal, intrapartum, and postnatal care services in underserved communities of Karachi.

PROJECT CONCENTRATED AREAS
PROJECT OBJECTIVES
To expand the integrated digital research platform across a wide range of marginalized communities in Karachi that will enable longitudinal data collection on pregnant women and children under the age of 2 years.

CAPLOW
VITAL Pakistan, a non-governmental organization, implemented an integrated maternal, newborn, and child health (MNCH) program using a continuum-of-care framework.

Implementation Duration:
(2014 - 2018)
PROJECT OVERVIEW
To simultaneously assess the impact on child mortality, we partnered with The Aga Khan University Department of Pediatrics, starting two years before the intervention period and continuing till the end of the intervention period. Between 2014 and 2018, under-5 mortality from 109 deaths per thousand live births to 64 deaths per thousand live births (41% reduction). The program resulted in an increase in the skilled delivery rate from 43% to 70%, increase in vaccination coverage up-to Penta-3 vaccine from 23% to 55%.
The program resulted in a 41% reduction in under-5 mortality over a 4-year period. Furthermore, there were notable improvements in the skilled delivery rate and vaccination coverage.
Program design:
- Quasi-experimental methods.
- Bi-monthly household surveillance.

PROJECT CONCENTRATED AREAS
The MNCH program is conducted in Karachi (Rehri Goth).

PROJECT OBJECTIVES
Scaling this program to a larger population and validating these results using more rigorous experimental has the potential to demonstrate a powerful model to reduce under-5 mortality in Pakistan and other low-and-middle-income countries.
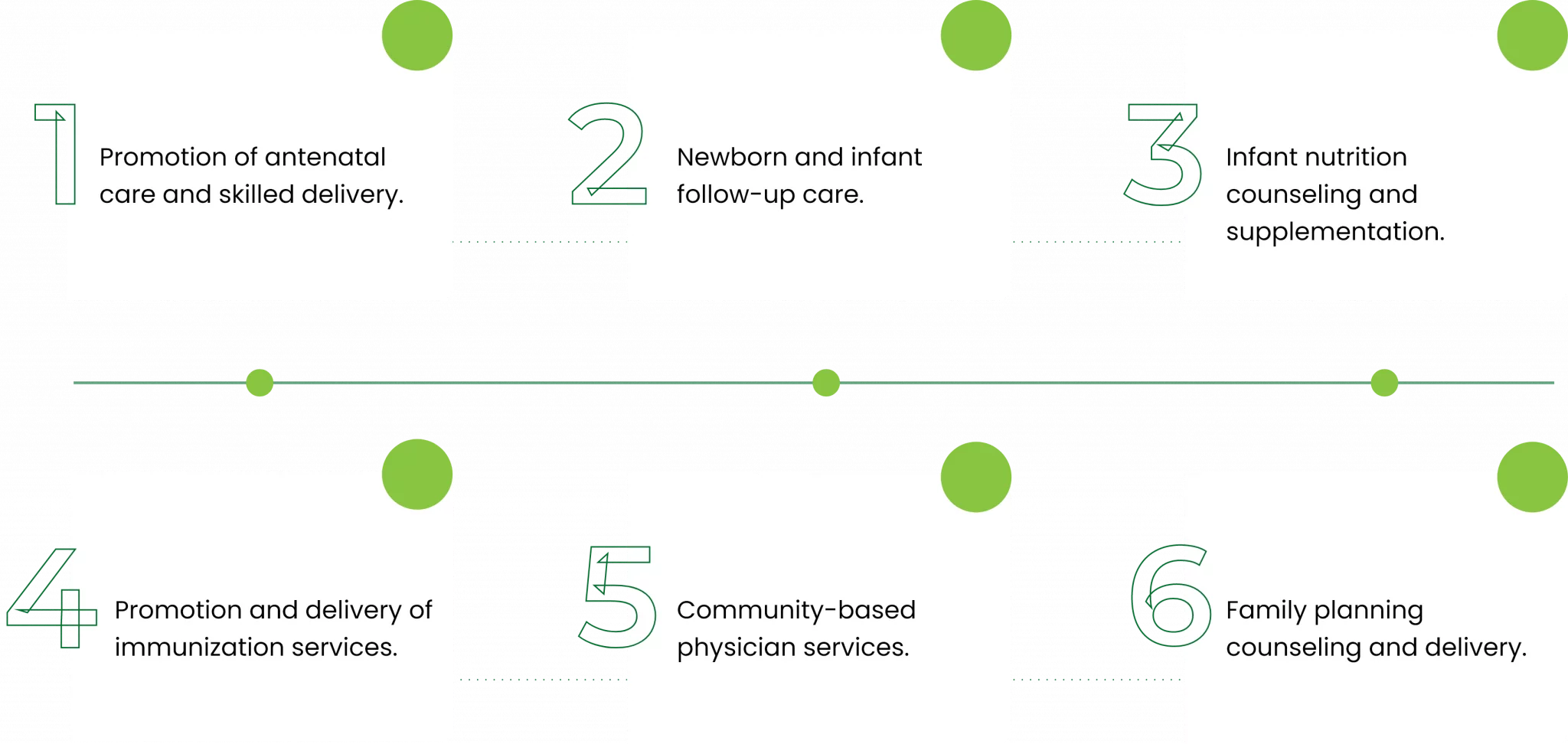
The MNCH package consisted of
MUMTA LW TRIAL
This project extensively covers the protocol of undernutrition maternal and perinatal trials.

Implementation Duration:
(2018-2020)
PROJECT OVERVIEW
Globally, 45% of under-five deaths are either directly or indirectly attributable to malnutrition, and most of these deaths are in low- and middle-income countries (LMICs). Children are particularly vulnerable in the first 6 months of life. An estimated 4.7 million infants under the age of 6 months are moderately wasted, whereas 3.8 million are severely wasted. Although the children of malnourished women have an increased risk of stunting and wasting, there is little information on this issue.
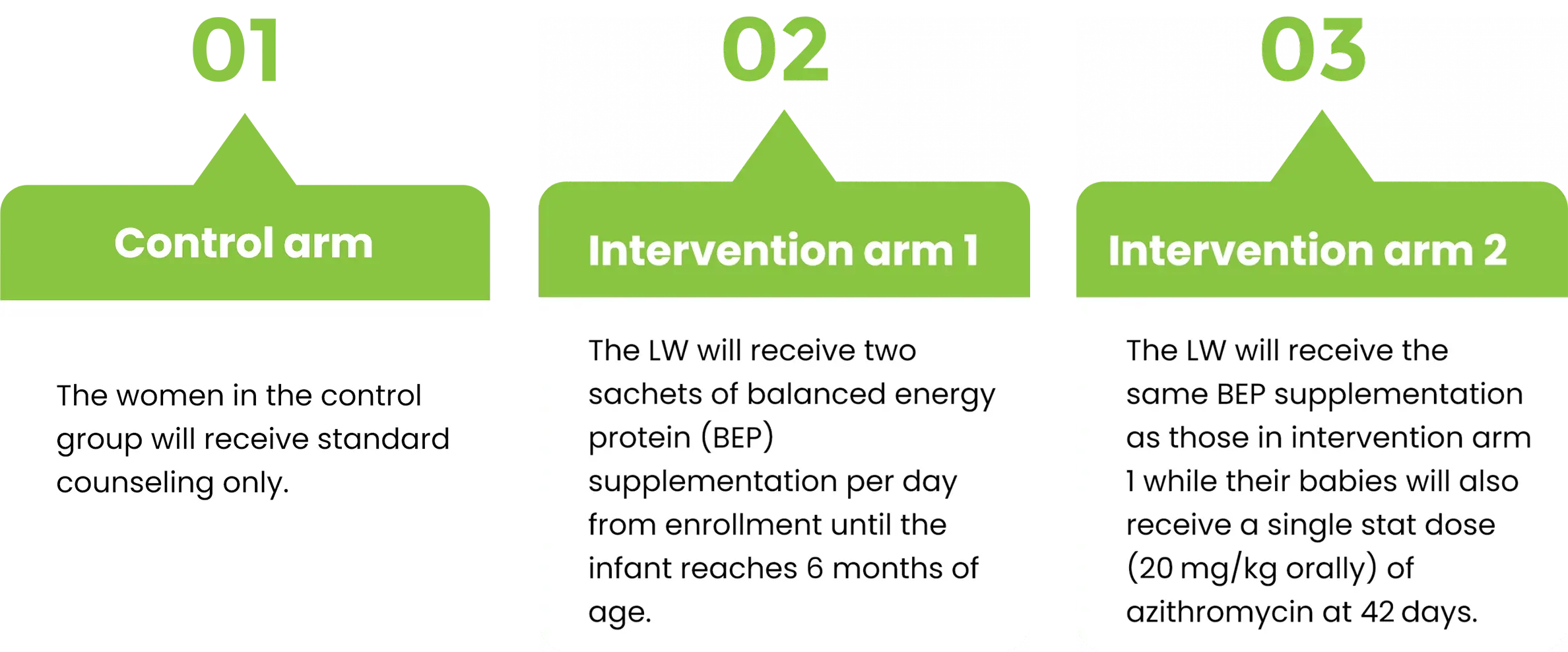
Trial design:
This is a community-based, open-label, multi-arm randomized controlled trial that will include parallel group assignments with a 1:1:1 allocation ratio in low-income squatter settlements in urban Karachi, Pakistan.

PROJECT CONCENTRATED AREAS
The trial is conducted in Ibrahim Hyderi and Rehri Goth.


PROJECT STUDY GROUPS
This trial-based project has the objective to aid undernutrition Pregnant Women with fundamental supplements.
MUMTA PW TRIAL
This project extensively covers the protocol of undernutrition maternal and perinatal trials.

Implementation Duration:
(2022 - 2025)
PROJECT OVERVIEW
Maternal undernutrition is critical in the etiology of poor perinatal outcomes and accounts for 20% of small-for-gestational-age (SGA) births. High levels of food insecurity, antenatal undernourishment, and childhood undernutrition necessitate the supplementation of fortified balanced energy protein (BEP) during pregnancy in low-income settings, especially with scarce literature available on this subject.
Trial design:
Prospective cohort trial:
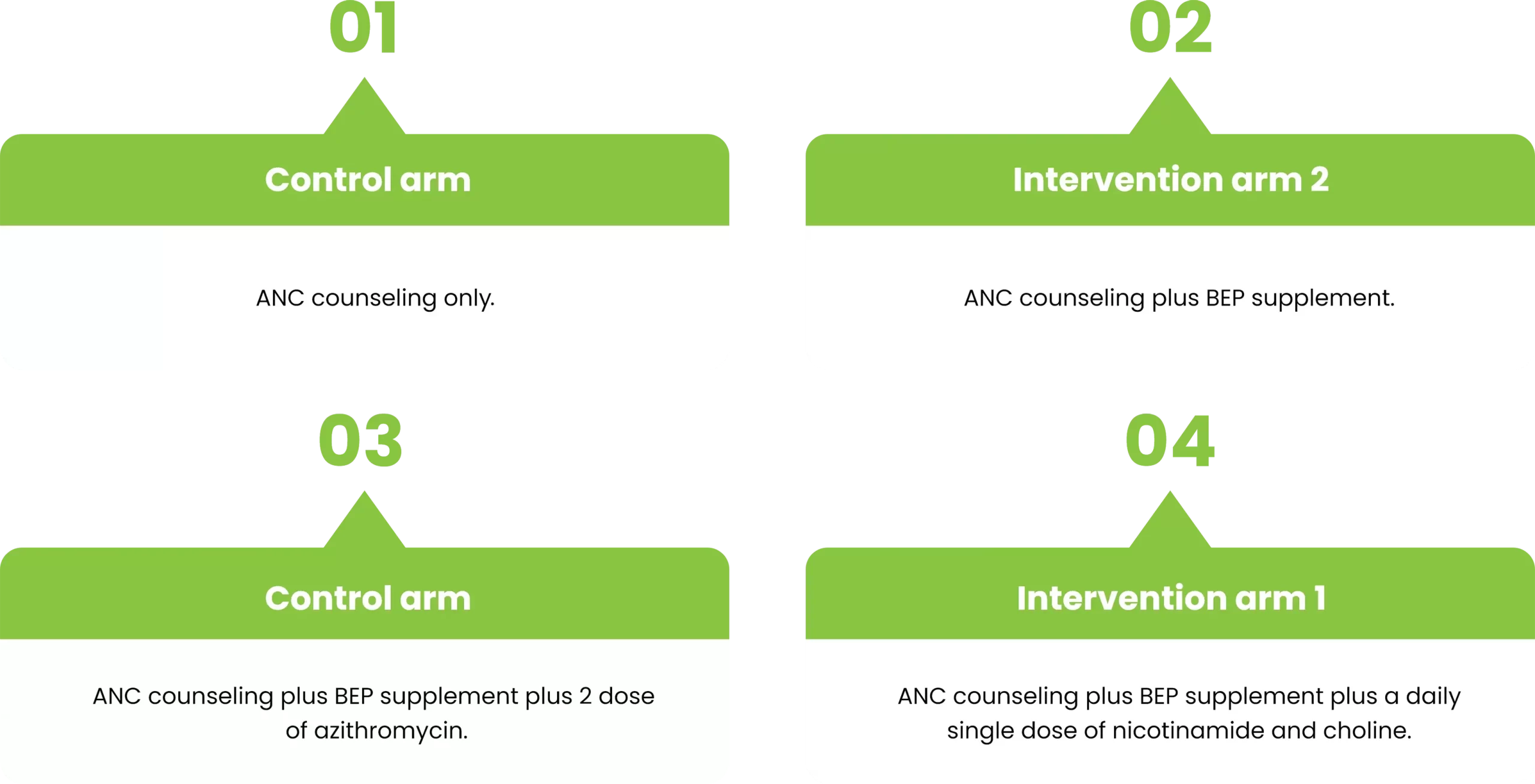
All pregnant women (PW), if identified between > 8 and < 19 weeks of gestation based on ultrasound, will be offered routine antenatal care (ANC) counseling and voluntary participation in the trial after written informed consent. A total number of 1836 PW will be enrolled with informed consent.
The trial is community-based, open-labeled, four-arm, and randomized controlled that will include parallel-group assignments with a 1:1:1:1 allocation ratio in low-income squatter settlements in urban Karachi, Pakistan.

PROJECT CONCENTRATED AREAS
The trial is conducted in Ibrahim Hyderi and Rehri Goth.


PROJECT STUDY GROUPS
This trial-based project has the objective to aid undernutrition Pregnant Women with fundamental supplements.

DONATE NOW
“All donations are directly benefitting programs that will ensure continued improvement in health care of women and children in under-privilege areas of Pakistan.”
Donate
